Matter shows many properties. Thermal properties are a part of those. Thermal physics deals with the temperature and other thermal properties of matter. Thermodynamics is one of the branches of thermal physics. It primarily deals with the concept of heat and temperature. It is also helpful in studying the interconversion of energy from one form to another.
Thermodynamics deals with macroscopic concepts of matter. This means that thermodynamics involves the study of bulk systems. It does not focus on the molecular constitution of matter. The study of thermodynamics also involves some other macroscopic concepts.
For example, when you describe a gas in microscopic terms you deal with the coordinates and velocities of molecules that constitute the gas. In comparison, thermodynamics deals with macroscopic concepts like the pressure, volume, temperature, mass, volume, etc. of the gas. Thermodynamics is not concerned with the motion of the system it deals with the internal macroscopic structure of the gas.
In mechanics, when the net external force and torque are applied to the system the situation is called equilibrium. In thermodynamics, equilibrium has a different context. When the different macroscopic variables that define the system do not fluctuate with time then the system is said to be in thermal equilibrium.
Consider a gas kept in a closed rigid container and insulated completely from the surroundings. The gas has fixed values of temperature, mass, volume, and composition which do not show changes with time. Then the gas is in thermal equilibrium.
The image shows heat flow to achieve thermal equilibrium.

Thermal equilibrium.
The state of equilibrium of the system is dependent on the surroundings and the nature of the wall that is separating the system from its surroundings. Take two games A and B. Assume the pressure of both gases as PA and PB and volume as VA and VB respectively. In the first case, you separate the two gases with the help of an Adiabatic wall (Which does not allow the flow of energy). In this situation, all the possible pairs of PA, and VA will be in equilibrium with PB, and VB.
But if you would have separated gases with a diathermic wall (A conducting wall that allows the flow of energy) then the results would have been different. In this condition, the macroscopic variables change spontaneously till they achieve equilibrium states. Once, they achieve the new equilibrium, the new variables of both gases will be in thermal equilibrium with each other. The concept of temperature in thermodynamics can be explained by the zeroth law of thermodynamics.
Zeroth Law of Thermodynamics
Consider two systems A and B separated via an adiabatic wall. Assume each one of them is in contact with a third system C via a conducting wall. The macroscopic variables will keep on changing till both systems A and B come into thermal equilibrium with C. After the thermal equilibrium is reached the adiabatic wall between A and B is replaced by a conducting wall. At the same time system, C is isolated from A and B via an adiabatic wall. According to the observations, there is no change in the state of A and B. This implies that both systems A and B are in thermal equilibrium with each other.
The above observation can be explained by the Zeroth law of thermodynamics. It states that “ two systems in a thermal equilibrium with a third system are in thermal equilibrium with each other”. This law was formulated by R.H. Fowler in 1931. The Zeroth law of thermodynamics was formed after the formulation of the first and second laws of thermodynamics.
The Zeroth Law of Thermodynamics states it clearly that when two systems are kept in thermal equilibrium with C a physical quantity must have a similar value for both. This physical quantity here is temperature.
Heat, Internal Energy and Work Done
With the help of the zeroth law of Thermodynamics, you got introduced to the concept of temperature. Temperature measures the hotness of a body. Temperature helps in determining the direction of heat flow when two bodies are kept in contact. Heat flows from the body at a higher temperature towards the body at a lower temperature. This flow of heat is stopped when the two bodies achieve a state of equilibrium.
Internal Energy: You know that bulk systems consist of a large number of molecules. The sum of the potential and kinetic energies of these molecules is known as Internal energy. Here, in thermodynamics molecular kinetic and potential energies are considered to calculate Internal energy.
The Internal energy of the system is denoted as U. Internal energy is only dependent on the final state of the system. It does not consider how that state was achieved. In simples terms, Internal energy is an example of a thermodynamic state variable. It is independent of the path taken by the system to achieve the state. The thermodynamic state variables include pressure, volume, temperature, and internal energy.
There are two ways to change the internal energy i.e. state of the system. Take into consideration a cylinder filled with a certain mass of the gas system and has a movable piston. One way to change the internal energy that you keep the cylinder in contact with a body at a higher temperature as compared to the temperature of the gas. Due to the temperature difference, the heat flow will take place. The flow of heat from hotter to colder end will change the internal energy of the system.
The other way to change the internal energy of the system is to push the piston down. This means that you are doing some work on the system. Heat and work are not the state variables of thermodynamics. They are used to transfer energy to a system which results in a change in the Internal energy of the system.
First Law of Thermodynamics
As discussed in the above section, the internal energy of the system can be changed by two methods of heat transfer that is heat and work. Assume:
∆Q= Heat supplied to the system by the surroundings
∆W= Work done by the system on the surroundings
∆U= Change in the internal energy of the system
Then, taking into consideration the general principle of energy conservation,
∆Q=∆U + ∆W, this means that the energy supplied to the system is consumed in increasing the internal energy of the system and the rest is consumed in doing work on the environment. This is the First Law of Thermodynamics. In sim,ple terms it is general energy conservation law. This law is applied to any system where the energy transfer from or to the surroundings is taken into consideration.
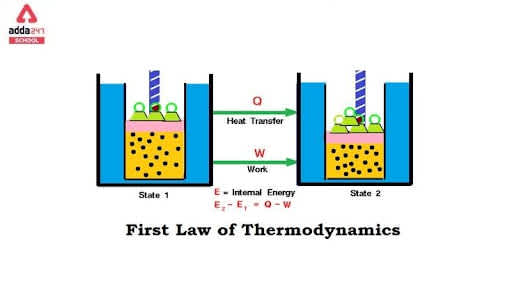
First Law of Thermodynamics
The equation for the first law of thermodynamics can be rearranged in many ways. Some of them are as follows:
To calculate the internal energy of the system: ∆U=∆Q - ∆W
To calculate heat energy by the thermodynamic state variables: ∆Q=∆U + P∆V or ∆Q=∆U + V∆P. In this equaltion the work done that is ∆W= is taken as P∆V ( pressure constant, volume variable) or as V∆P ( volume constant, pressure variable). Also if in the above equation the change in internal energy is zero then, ∆Q=∆W. That is the heat energy is equal to the work done or the entire heat energy is consumed.
Specific Heat Capacity
Specific heat for the any substance is defined as the amount of heat energy required to change the temperature of 1gram of substance by 1 degree Celsius. To determine the value for the specific heat capacity of any substance two materials who are at different temperatures initially are placed in contact with one another. The heat flows from the hotter end to the colder end. You know that when the substance absorbs heat energy its temperature rises. When you give same amount of heat to equal mass of different substances you can notice that the rise in temperature for each substance will be different. This is because each substance has got different heat capacities. The heat required to raise the temperature of the whole system is known as heat capacity and the heat required to change the temperature by 1 degree Celsius of 1gm of substance is called a s specific heat capacities. The SI unit of specific heat capacity is JK -1. The formula for specific heat capacity is:
Q= Cm∆T, where Q= Heat quantity absorbed by the system
m= Mass of the body
∆T= Rise in temperature
C= specific heat capacity of substance which depends on nature of the material and substance.
Specific Heat Capacity Of Water
Specific heat capacity for water can be described as the amount of heat energy required to change the temperature of 1 litre of water by one degree Celsius. That is from 14.5 degrees Celsius to 15.5 degrees Celsius.
In the case of gases, you can describe two specific heat capacities that are the specific heat capacity at constant volume and specific heat capacity at constant pressure. In the case of ideal gases, this can be explained by a simple relation known as Mayer Relation. This relation can be stated as CP – CV= R.
In the above relation, CP and CV are the molar-specific heat capacities of an ideal gas at constant pressure and volume. And R is the universal gas constant.
Thermodynamic State Variables and Equation of State
When the temperature of the surroundings is equal to the temperature of a system that condition is called thermal equilibrium. The variables that describe the thermodynamic equilibrium are referred to as thermodynamic state variables. The thermodynamic state variables are dependent only on the state of the system. They do not associate with the path taken by the system to achieve that state of equilibrium.
The equation of state is the relationship between the state variables of thermodynamic equilibrium. In the case of an ideal gas, this equation can be written as PV= µRT.
In the case of real gas this equation can be written as: [P + a/V2] [V-B] = µRT. This is called Vander wall’s Gas Equation.
Thermodynamic state variables are of two types extensive state variables and intensive state variables. A variable that is independent of the size of the system is known as the extensive variable. A variable that is independent of the size of the system and also has got a uniform value in the varied subdivision of the system is called an intensive state variable. In any equation of thermodynamics quantities present on both sides have to be either extensive or intensive.

Quasi-Static Processes In Thermodynamics
Slow processes in thermodynamics are referred to as Quasi-Static Processes. These processes occur very slowly. During the quasi-static processes, all the states are in equilibrium. The process during which the system is in thermodynamic equilibrium with the surroundings during the entire duration is called a quasi-static process.
Thermodynamic processes
A thermodynamic process is described as a sequence of various processes that begins and ends at a similar thermodynamic state. There are four major thermodynamic processes. These include:
Isothermal processes: If the temperature of the system remains constant when it changes from one state to another it is called an isothermal process. If in any thermodynamic problem it is mentioned isothermal then it implies constant temperature.
Isobaric process: If the pressure of the gas remains constant when it changess its state then this situation is referred to as Isobaric pressure,
Isochoric process: If the volume of the system stays fixed during its transition from one state to another it is called an isochoric process.
Adiabatic process: If the heat content of the substance or the quantity of the matter stays fixed during its transformation from one state to another it is called an adiabatic process. There is no exchange of heat between the system and surroundings in the case of an adiabatic process.
These are the four quasi-static processes that take place in thermodynamics.
Heat Engine
A heat engine is useful in converting heat into work. The heat engine takes the heat from the reservoir which is a hot body for carrying out work. Some of the haet are discharged into the sink which is a cold body. Some form of energy is wasted as heat. There are two major types of heat engines that are:
External combustion engine
Internal combustion engine
Working of heat engine
A heat engine majorly has three parts that are a heat reservoir, an engine, and a cold sink. The heat produced during external or internal combustion is supplied to where the piston moves. The power generated is supplied to the machine which is connected to the engine and hence the work is done. All the excess heat is transferred to the sink. The temperature of both the reservoir and sink stays the same.
The efficiency of heat engine
The efficiency of any device is the fraction of heat given as input at a high temperature that changes to work. According to the second law of thermodynamics, no engine can have 100% efficiency.
The efficiency of the Carnot engine is given as,
Η =Work done / Heat input
We know that,
Work done, W = Q1 -Q2
Heat input =Q1
Then,
Efficiency, η =W / Q1
=(Q1-Q2) /Q1
=1-(Q2 /Q1), which is the efficiency of the heat engine.
If Q2 = 0, then efficiency = 100%. This is known as the Carnot engine.
It is an ideal case where the efficiency is 100%. However, there will be some loss of energy in the system and hence, for every engine, there will be a limit of efficiency.
Meanwhile, it is also known that,
Thermodynamically, (Q2/Q1) = (T2/T1)
Therefore,
Η = 1 – (T2/T1)
Work done in each step:
Isothermal expansion:
Wa → b = Q1= n R T 1 ln(v2 / v1)
Adiabatic expansion:
Wb → c = ( nR /( γ -1))(T1-T2)
Isothermal compression:
Wc → d = nRT2ln(v3/v4)
Adiabatic compression:
Wd → a = ( nR /( γ -1))(T1-T2)
Hence, the total work done by the gas on the environment in one complete cycle is given by, W= Wa → b+Wb → c+Wc → d +Wd → a
Net efficiency = (net work done by the gas) / (heat absorbed by the gas)
=W / Q1
=(Q1-Q2) / Q1
=1-(Q2 / Q1)
Refrigerators
Heat engine is used to convert the heat energy into work. Refrigerator is a device which works exactly opposite of the heat engine. In the refrigeration cycle five components play role that includes fluid refrigerant, condenser coil, compressor, evaporator coil, and expansion device. The compressor constricts the vapour from the refrigerant and thus the pressure increases. Due to this the vapour is pushed in the coils placed outside the refrigerator. The refrigerant inside the fridge absorbs the heat and cools down the air slowly. In the end the refrigerant evaporates and then it flows back to the compressor and thus the cycle starts repeating itself.
Second Law of Thermodynamics
According to the second law of thermodynamics any process that is occurring spontaneously will lead to an increase in the entropy of the surroundings. In other words, it explains that there will be no decrease in the entropy of an isolated system with time.
In some of the cases, if the system is in thermodynamic equilibrium or is undergoing a reversible process the overall entropy of the system as well as the surroundings remains constant. The second law states the fact that it is almost impossible to convert the heat energy into mechanical energy with 100% efficiency.
Mathematically second law of thermodynamics can be written as:
∆Sun > 0. Here ∆Sunis the change of entropy in the universe.
There are two statements given for the second law of thermodynamics: The kelvin-Plank Statement and the Clausius statement.
Plank Statement: No heat engine can produce a network in a complete cycle if it is exchanging with bodies kept at a constant or fixed temperature.
Clausius Statement: There is no process where 100% of the heat energy can be transferred from the colder body to the hotter body. Heat pumps and refrigerators are devices that work on this statement.
Reversible and Irreversible processes
There are two major processes in thermodynamics which include reversible processes and irreversible processes:
Reversible Process: A thermodynamic process can be turned back to a situation where both the system and surroundings return to their original positions. Ideally, there is no such process. Thus they can be called assumptions based on which the limitations of any devices are decided. Examples: Slow adiabatic compression or expansion, Slow isothermal expansion or compression of gases
Irreversible process: These processes are the opposite of reversible processes. Under irreversible situations, the system and surroundings fail to return to their original states. Examples: Heat transfer, diffusion.
Carnot engine
A Carnot engine is called an ideal engine. It operates under a closed and reversible thermodynamic system. The working substance undergoes four processes two expansions and two compressions. These include isothermal and adiabatic expansion and isothermal and adiabatic compression. Many other thermodynamic applications work on this basis and also have very vast applications.
Even though a Carnot engine is considered an ideal engine still the theoretical efficiency is not achieved. This is because of friction. The Carnot engines efficiency is dependent on the temperatures of hot and cold reservoirs.
In a Carnot engine heat, Q1 is taken from the source and heat Q2 is rejected from the sink. During this, the work done is expressed as W= Q1 – Q2. The complete cycle of working Carnot is described below:
Isothermal expansion: During this process, the temperature of source T1 is used to draw Q1 amount of heat from the source. As the process is isothermal expansion so the overall change in internal energy is zero. The heat absorbed by the gas is equal to the work done by the gas on the environment.
Adiabatic expansion: During the process of adiabatic expansion of an ideal gas there is a fall in the temperature of the ideal gas. It falls from the temperature of the source T1 to the temperature of the source T2. As there is no supply of heat energy the pressure of the gas falls and there is a rise in the volume of the gas. The work done on the gas is less and so the pressure on the gas should be below.
Isothermal Compression: As the process of isothermal compra session for an ideal gas begins the sink temperature T2 further falls. This is because it rejects heat Q2 to the sink.
Adiabatic Compression: When the ideal gas undergoes adiabatic compression the temperature of the gasses automatically from T2 to T1. Due to this temperature rise, the working substance returns to its original state thus completing the cycle.
Conclusion
This was all about thermodynamics. Now you would have to understand that thermodynamics is a branch of physics that deals with the exchange of heat in the form of work.
Many laws explain the processes that occur in thermodynamics known as laws of thermodynamics. Also, many devices work on applications and principles of these laws.
