The second law of thermodynamics states that the energy is moved or changed, increasingly and more of it will lose. It's one of the four laws of thermodynamics, which depict the connections between nuclear power, or heat, and different types of energy, and what energy means for the issue.
second-law-banner
The second law of thermodynamics shows limitations upon the bearing of heat move and achievable efficiencies of heat motors. The principal law of thermodynamics expresses that the energy of the universe stays consistent however, the energy can be traded among framework and environmental factors, it can't be made or destroyed. While the primary law of thermodynamics gives data about the amount of energy moving as a cycle, it neglects to give any bits of knowledge about the course of energy move and its nature. The principal regulation can't show whether a metallic bar of uniform temperature can unexpectedly become hotter on one side and cooler on the other. Everything that could be expressed is that there will continuously be energy balance assuming the cycle happens. The second law of thermodynamics gives the rule to the likelihood of any interaction. A cycle can't happen except if it fulfills both the first and second laws of thermodynamics.

entropy
According to the second law of thermodynamics, in straightforward terms, entropy generally increases. This standard makes sense of, for instance, the way you can't unscramble an egg.
In a reversible process, a minute development in the entropy (dS) of a shut framework (which permits the passage or exit of energy - yet not move of issue) is characterized to result from a microscopic exchange of heat (δQ) to or from the framework, partitioned by the normal temperature (T) of the framework in balance and the environmental factors which supply or take up the heat Various documentations are utilized for a microscopic measure of heat (δ) and little difference in entropy (d) since entropy is an element of the state, while heat, similar to work, isn't. For a little cycle without trade of mass with the environmental elements, the subsequent regulation expects that the development in framework entropy satisfies the inequality This is because a general process for this case may include work being done on the system by its surroundings, which can have frictional or viscous effects inside the system, because a chemical reaction may be in progress, or because heat transfer occurs only irreversibly, driven by a finite difference between the system temperature (T ) and the temperature of the surroundings Note that the equality still applies for pure heat flow,

formula-1
which is the premise of the precise assurance of the outright entropy of unadulterated substances from estimated heat limit bends and entropy changes at stage advances, for example by calorimetry. Introducing a bunch of inner factors to depict the deviation of a thermodynamic framework in actual harmony (with the necessary distinct uniform strain P and temperature T) from the compound balance state, one can record the balance
The subsequent term addresses the work of interior factors that can be annoyed by outer

formula-2
impacts; however, the framework can't play out any certain work through inward factors. This assertion presents the difficulty of the inversion of development of the thermodynamic framework on schedule and can be considered as a plan of the second standard of thermodynamics, the definition, which is, obviously, comparable to the detailing of the rule as far as entropy.
The zeroth law of thermodynamics in its typical short articulation permits acknowledgment that two bodies in a connection of warm harmony have a similar temperature, particularly that a test body has a similar temperature as a kind of perspective thermometric body. For a body in warm balance with another, there are endlessly numerous experimental temperature scales, overall, individually relying upon the properties of a specific reference thermometric body. The second regulation allows a recognized temperature scale, which characterizes a flat-out, thermodynamic temperature, autonomous of the properties of a specific reference thermometric body.
What is the Second Law of Thermodynamics?
The second law of thermodynamics expresses that any precipitously happening cycle will continuously prompt an acceleration in the entropy (S) of the universe. In straightforward words, the law makes sense that a separated framework's entropy won't ever diminish after some time.
Regardless, sometimes when the framework is in thermodynamic balance or going through a reversible cycle, the absolute entropy of a framework and its environmental factors stays consistent. The subsequent regulation is otherwise called the Law of Increased Entropy
The subsequent regulation makes sense that it is difficult to change heat energy over to mechanical energy with 100% ability. For instance, assuming that you take a gander at the cylinder in a motor, the gas is warmed to expand its strain and drive a cylinder. Notwithstanding, even as the cylinder moves, there is generally some extra heat in the gas that can't be utilized for completing some other work. Heat is squandered and it needs to be disposed of. For this situation, it is finished by moving it to a heat sink or on account of a motor, squander heat is disposed of by debilitating the pre-owned fuel and air combination to the environment. Moreover, the heat produced from erosion that is for the most part unusable ought to likewise be eliminated from the framework.
Second Law of Thermodynamics Equation
Numerically, the second law of thermodynamics is addressed as; ΔSuniv > 0 ΔSuniv is the adjustment of the entropy of the universe. Entropy is the proportion of the direction of the framework or we can say that it is the proportion of energy or chaos inside a confined framework. It may be considered a quantitative record that portrays the nature of the energy. In the meantime, there are not many elements that cause an expansion in entropy of the shut framework. In a shut framework, while the mass remaining parts are consistent there is a trade of heat with the environmental factors. This adjustment of the heat content worsens the framework accordingly expanding the entropy of the framework. Besides, inside changes might happen in the development of the particles of the framework. These prompts worsen which further causes irreversibility inside the framework bringing about the addition of its entropy.
Different Statements of The Law
There are two explanations for the second law of thermodynamics they are;
*Kelvin- Plank Statement
*Clausius Statement
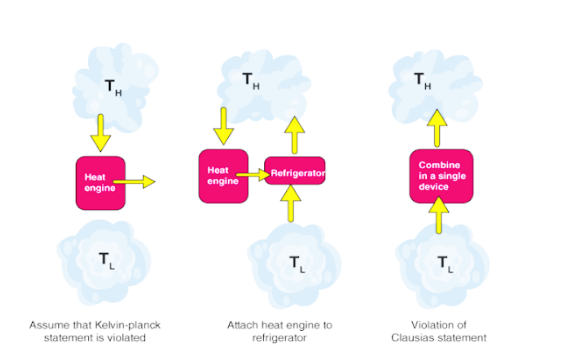
Kelvin-Planck Statement
It is outside the realm of possibilities for a heat motor to create an organization in a total cycle on the off chance that it trades heat just with bodies at a solitary fixed temperature.
Special cases:
If Q2 =0, the heat motor produces work in a total cycle by trading heat with just a single supply, subsequently disregarding the Kelvin-Planck articulation.
Clausius’s Statement
It is difficult to develop a gadget working in a cycle that can move heat from a colder body to a hotter one without consuming any work. Likewise, energy won't stream immediately from a low-temperature object to a higher temperature object. It is critical to take note that we are alluding to the net exchange of energy. Energy move can occur from a virus object to a hot article by a move of fiery particles or electromagnetic radiation.
Notwithstanding, the net exchange will happen from the hot item to the virus object in any unconstrained cycle. What's more, some type of work is expected to move the net energy to the hot article. All in all, except if the blower is driven by an outside source, the fridge will not have the option to work. The heat siphon and cooler work on Clausius' articulation

formula-3
Both Clausius' and Kelvin's proclamations are comparable i.e a gadget disregarding Clausius' explanation will likewise abuse Kelvin's assertion as well as the other way around.
formula-3
French physicist Nicolas Léonard Sadi Carnot who was called the "father of thermodynamics," fundamentally presented the Second Law of Thermodynamics. Notwithstanding, according to his assertion, he underscored the utilization of the caloric hypothesis for the portrayal of the law. Caloric (self-anti-agents liquid) connects with heat and Carnot saw that some caloric was lost in the movement cycle.
Statistical mechanics
Factual mechanics clarify the subsequent regulation by hypothesizing that material is made out of particles and atoms which are the inconsistent movement. A specific arrangement of positions and speeds for every molecule in the framework is known as a microstate of the framework and as a result of the steady movement, the framework is continually changing its microstate. Measurable mechanics hypothesizes that, in harmony, each microstate that the framework may be in is similarly liable to happen, and when this supposition is made, it drives straightforwardly to the end that the subsequent regulation should hold from a factual perspective. That is, the subsequent regulation will hang by and large, with a measurable minor departure from the request for 1/√N where N is the number of particles in the framework. For regular (naturally visible) circumstances, the likelihood that the subsequent regulation will be abused is zero. Nonetheless, for frameworks with few particles, thermodynamic boundaries, including the entropy, may show huge measurable deviations from that anticipated continuous regulation. The traditional thermodynamic hypothesis doesn't manage these measurable varieties.
The Inequality of Clausius
The Carnot cycle is a reversible cycle working between two supplies. Assuming an irreversible cycle works between similar two repositories, the accessible work from the irreversible cycle should be not exactly the accessible work from the reversible cycle. That is

formula-3
If the primary regulation is applied to a cycle and equivalent measures of heat QH are moved from the high-temperature supply, more heat will be moved to the low-temperature repository for the irreversible cycle

forumla-4
So when you perform the cyclic integral for dS.

forumla-4
For a reversible interaction, the above necessary is, obviously, 0. Assuming that an irreversible fridge is being viewed as more work will be expected than for a reversible cooler, the heat moved at the high-temperature repository will be more noteworthy so the cyclic essential will in any case be under zero for an irreversible interaction. So the principal result can be composed for any cycle

formula-5
This is called the inequality of Clausius and it is a consequence of the second law of thermodynamics.
The second law in chemical thermodynamics
For an unconstrained substance process in a shut framework at steady temperature and tension without non-PV work, the Clausius imbalance ΔS > Q changes into a condition for the adjustment of Gibbs free energy

forumla-6
In principle, a few connections, for example, crashes of unbending bodies or certain substance responses, look similar whether they are run forward or in reverse. Practically speaking, notwithstanding, all trades of energy are dependent upon shortcomings, for example, erosion and radiative hotness misfortune, which increment the entropy of the framework being noticed, as per OpenStax.
Consequently, because there is no such thing as a reversible cycle, assuming somebody requests what is the bearing from time, we can reply with certainty that time generally streams toward expanding.