Heat is an energy that is moved starting with one body then onto the next as the aftereffect of a distinction in temperature. Assuming two bodies at various temperatures are united, energy is moved i.e., heat streams from the more sweltering body to the colder. The impact of this exchange of energy as a rule, yet not forever, is an expansion in the temperature of the colder body and a reduction in the temperature of the more blazing figure.
A substance might assimilate heat without an expansion in temperature by changing from one actual state (or stage) to another, as from a string to a fluid (liquefying), from a string to a fume (sublimation), from a fluid to a fume (bubbling), or starting with one strong structure then onto the next (normally called glasslike progress). The significant differentiation between heat and temperature (heat being a type of energy and temperature a proportion of how much that energy is present in a body) was explained during the eighteenth and nineteenth hundreds of years.
Source of the heat
There are many sources of heat but the following are the main sources of heat:
Sun
Chemical
Electrical
Nuclear
What is heat?
The vast majority of us prefer the word 'heat' to anything that feels warm yet deductively, heat is characterized as the progression of energy from a warm to a cooler article. The classification of heat is done on this premise as hot and cold. Heat energy is surrounding us, for example, in icebergs, volcanoes, and our bodies. Each of them has heat energy. The consequence of the development of moment particles known as atoms, molecules, or ions in fluids, solids, and gasses is heat energy. Heat energy can be moved from one substance to another and the flow of the temperature between two items is known as heat.
Three Mechanisms of heat transfer:

Three Mechanisms of heat transfer
Radiation
Warms the air utilizing heat waves that emanate out of the hot article every which way until consumed by different items. Heat moved by radiation happens at the speed of light and ventures significant stretches.
Conduction
Transfers heat from one object to another when in direct contact. The traveling molecules of a warm object can increase the energy of the molecules in a cooler object. Solids conduct heat more than liquids and gasses since c are close together.
Convection
Moves heat energy using air and fluids. The particles move separated and become less thick as the air warms up and subsequently makes the air rise. While cooler air moves in from underneath and warms up.
Heat as a form of energy

Heat as a form of energy
Since each of the many types of energy, including heat, can be changed over into work, measures of energy are communicated in units of work, for example, joules, foot-pounds, kilowatt-hours, or calories. Precise connections exist between the measures of heat added to or eliminated from a body and the magnitude of the impacts on the condition of the body. The two units of heat most ordinarily utilized are the calorie and the British warm unit (BTU). The calorie (or gram-calorie) is how much energy is expected to raise the temperature of one gram of water from 14.5 to 15.5 °C; the BTU is how much energy is expected to raise the temperature of one pound of water from 63 to 64 °F. One BTU is around 252 calories. The two definitions indicate that the temperature changes are to be estimated at a steady strain of one climate because the measures of energy included depending to a limited extent on the pressure. The calorie utilized in estimating the energy content of food varieties is the huge calorie, or kilogram-calorie, equivalent to 1,000 gram-calories.
By and large, how much energy is expected to raise a unit mass of a substance through a predefined temperature stretch is known as the heat limit, or the particular heat, of that particle. The amount of energy important to raise the temperature of a body one-degree changes relying on the limitations forced. Assuming heat is added to a gas restricted at a consistent volume, how much heat is expected to cause a one-degree temperature climb is not exactly if the heat is added to similar gas allowed to extend (as in a chamber fitted with a versatile cylinder) thus taking care of the business. In the main case, all the energy goes into raising the temperature of the gas, yet in the subsequent case, the energy not just adds to the temperature increment of the gas yet additionally gives the energy important to the work done by the gas on a container. Thus, the particular fierceness of a substance relies upon these circumstances. The most generally resolved explicit warms are the particular heat at steady volume and the particular heat at a constant tension. The heat limits of numerous strong components were demonstrated to be firmly connected with their nuclear loads by the French researchers Pierre-Louis Dulong and Alexis-Thérèse Petit in 1819. The alleged law of Dulong and Petit helped decide the nuclear loads of specific metallic components, yet there are numerous exemptions for it; the deviations were subsequently observed to be logical based on quantum mechanics.
It is wrong to talk about the heat in a body since it is confined to energy being moved. The energy put away in a body isn't heat (nor is it work, as work is likewise energy on the way). It is standard, notwithstanding, to discuss reasonable and inactive heat. The inert heat, likewise called the heat of vaporization, is how much energy is important to change the fluid to fume at consistent temperature and strain. The energy expected to liquefy a strong to a fluid is known as the heat of combination, and the heat of sublimation is the energy important to change a strong straightforwardly to a fume, these progressions additionally occur under states of steady temperature and tension. Air is a combination of gasses and water fumes, and it is feasible for the water present in the air to change stage; i.e., it might become fluid (downpour) or strong (snow). To recognize the energy related to the stage change (the dormant heat) and the energy expected for a temperature change, the idea of reasonable heat was presented. In a combination of water fume and air, reasonable heat is the energy important to deliver a specific temperature change barring any energy expected for a stage change.
Collision In Particles
Particles are having higher energy at higher temperatures. Some measure of this energy can be sent to different particles that are at a lower temperature. For example, when a quick voyaging molecule slams into a slower molecule in the gas state, it moves its energy to the next molecule and hence speeds up sluggish matters. Whenever billions of particles slam into one another, a locale of high energy moves across the material until a state of warm harmony is created, for example, the temperature across the material is something alike.
Sign Conventions for Heat Energy Transfer
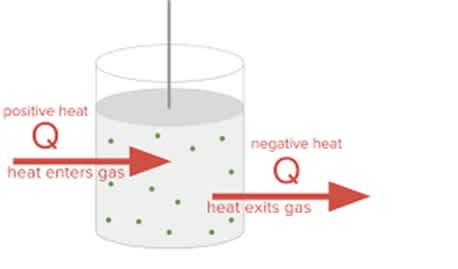
Sign Conventions for Heat Energy Transfer
In actual conditions, how much hotness moves is generally meant by the image Q. Heat move might be demonstrated by either a positive or negative number. The heat that is delivered into the environmental factors is composed of a negative amount (Q < 0). At the point when hotness is ingested from the environmental elements, it is composed as a positive worth (Q > 0).
Classification of Heat
Heat can be classified into two:
Hot
Cold
Hot
Objects with high heat content are characterized as hot (heat or cold of an article is an overall term that is generally estimated concerning a reference object). Instances of blistering articles around us incorporate the sun, fire, warm container, air from a hairdryer, magma from volcanic emissions, and so forth
Cold
Objects with lower heat content are characterized as cold items. The heat or coolness of an item is an overall term that is generally estimated concerning a reference object. Instances of cold items around us incorporate ice, air from a forced-air system, cold beverages, metal vessels kept open on cool cold weather days, and so forth
Distinction Between Heat and Temperature
Heat
Definition: Heat is characterized as the absolute energy of an article that has sub-atomic movement inside it.
SI unit: Joule
Symbol: Q
Temperature
Definition: Temperature is characterized as the proportion of the nuclear power of an item
SI unit: Kelvin
Symbol: T
Thermal Expansion

Thermal Expansion
The heat extension happens in solids, fluids, and gasses. Practically all substances grow when their temperatures increment, except if they are obliged in some way. Models that remember the warming of the air for a sight-seeing balloon, cause the inflatable to grow, rise, and mercury in a thermometer, which ascends in light of heat. Metal poles are utilized in an assortment of uses moreover. For instance, metal poles or strips that are utilized as extension joints at the finishes of scaffold areas represent the development of steel spans in a more smoking climate. How much extension that happens and how we foresee it relies upon the matters. For instance, a strong metal bar for the most part extends directly and expands long, while fluids and gasses experience an expansion in volume. In every one of the three cases, warm development happens in light of an expansion in temperature and helpful gadgets exploit this idea.
Thermodynamics
Thermodynamics is the investigation of heat and its change to mechanical energy. There are four laws of thermodynamics, however, we just focus on the two head regulations here: the primary regulation and the subsequent regulation. The main regulation says that the adjustment of the inward energy of a substance approaches the work done on it in addition to the heat moved to it. Numerically, we utilize the condition:
delta U = work + Q
Inside energy is the amount of the active and likely energies of the multitude of iotas and atoms inside the particles. The meaning of the main law of thermodynamics is that there are two methods for expanding the temperature of a substance:
1) By presenting it to another substance that has a higher temperature and
2) By doing specific sorts of work on the substance
Grating and pressure of gasses are two instances of ways of expanding temperature by technical work. Cylinders in gas-powered motors exploit this idea. Air is compacted in a chamber by the cylinder, which raises the temperature to right multiple times the temperature of the uncompressed state.
The subsequent regulation says that heat can't be moved from a colder body to a more sizzling body without work being finished by an external specialist. Expressed another way, no gadget can be constructed that will over and over remove heat from a source and convey mechanical energy without shooting heat to a lower-temperature supply. The ideal model is the heat motor, which is examined later in this example.
In conclusion, we can say that:
Taking everything into account, the exchange of heat or nuclear power will ordinarily change the temperature of the substance, but not frequently for instance, right when the ice in the bowl goes to water those water atoms will be at precisely the same temperature as when they were ice.
