ENERGY The word energy is used commonly in daily life. The whole matter possesses energy. Energy exhibits itself in so many different forms. What is energy? The energy of a body is its strength or capacity of the body to do work. Energy is a scalar quantity as it has a magnitude but no direction. The whole quantity of energy in the universe is constant. Energy can be conserved and transferred to other matter. Energy can be transformed from one form to another and the amount of energy transformed can be measured. Image: This blog deals with ENERGY. Here, you can learn about what is kinetic energy and potential energy? And other different forms of energy.
When a body is capable of performing some work, it is said to possess energy. The dimensions of energy are [M1 L2 T-2] where M = Mass, L= Length, and T = Time. The SI unit of energy is Jouleand in the cgs system, the unit of energy is erg.
Energy can neither be created nor be destroyed, but energy can only be transferred or transformed from one material to another. The transfer of energy from one location to another is known as energy transfer. The conversion of energy from one form to another is known as energy transformation. Whether energy transfers or transforms, the total amount of energy never changes which is called energy conservation.

energy-banner
THE PRINCIPLE OF CONSERVATION OF ENERGY
The law of conservation of energy states that in a closed system that is isolated from its surroundings, the total energy of the system does not change.Taking all kinds of energy into account, the total energy of an isolated system often remains constant. In simple words, in any isolated system if there is a loss of energy somewhere there must be a gain of an equal amount of energy in another part of it. There is no example known of disobeying this law. In any system, the amount of energy is determined by the following equation:-
Every form of energy obeys the law of conservation of energy. The total energy of all kinds in the universe remains constant at all times only changing from one form to another. Thus, Energy can neither be created nor be destroyed.
Some energy transfers can be heard, felt, seen, or even tasted. Energy is used in many forms in daily lives. Interestingly, everything work done in day to day activities is somehow related to energy. For example:-
In the batteries of the torch, the electric energy is being changed into light and heat energy.
In the loudspeakers, the electrical energy is converted into sound energy.
When fuels are burnt, the chemical energy is being transformed into light or heat energy.
In winters we rub both our hands together for warmth, which involves kinetic energy converting into thermal energy.
Different Forms of Energy
So, there are different forms of energy that are used in daily life but they are not known well. Some of the forms of energies include:
Energy manifest itself in so many different forms:-
KINETIC ENERGY
Kinetic energy is a form of energy that is associated with the motion of an object. The energy possessed by a body by virtue of its motion.Kinetic energy is a scalar quantity and is entirely defined by magnitude alone. The absolute unit of kinetic energy is a joule.
Examples of kinetic energy:-
Windmills work with the help of the kinetic energy of air.
Sailing ships consume the kinetic energy of wind.
Watermills work on the kinetic energy of water.
![types-of-energy]()
types-of-energy
The formula of kinetic energy is KE =½mv². Thus, the kinetic energy of a body is half of the product of the mass of the body and the square of the velocity of the body.
The kinetic energy of a body can be obtained either from
The amount of work done in stopping the moving body.
The amount of work done in moving the body from the state of rest.
The kinetic energy of a body having non-uniform circular movement is not constant. For example, when a body is moving in a vertical circle, its K.E is maximum at the lowest point and minimum at the highest point.
RELATION BETWEEN KINETIC ENERGY AND LINEAR MOMENTUM
Let m= mass of a body
v = velocity of a body
Linear momentum of the body p=mv -(1)
And Kinetic energy of the body = ½ mv² -(2)
Putting the value of linear momentum (equation-1) in equation(2).
Therefore, K.E = p²/2m
This is a significant relation that shows that a body cannot have K.E without having linear momentum and its reverse is also true.
Figure (a)

realation-kw-lm
If p=constant, K.E is inversely proportional to the mass of the body.
Figure (b)

realation-kw-lw-2
If K.E = constant, p²∝ m
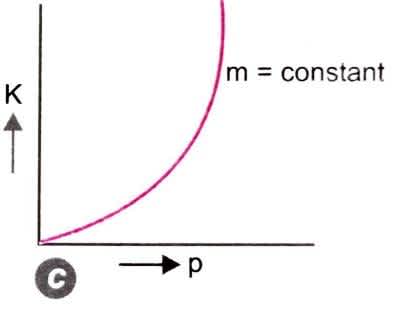
Figure (c)
realtion-kw-lm-3
If m=constant, p²∝ K.E
WORK ENERGY PRINCIPLE
According to the work-energy principle, work done by the net force in displacing a body is equal to a change in the kinetic energy of the body. Thus, when a force does some work on a body, the kinetic energy of the body rises by an equal amount.
Work done = Final kinetic energy of the body – Kinetic energy of the body
W = Kf -Ki = increase in kinetic energy of the body.
According to the work-energy principle, work and kinetic energy are similar quantities.
POTENTIAL ENERGY:-
The potential energy of a body is defined as the energy possessed by the body by virtue of its position or composition in some region. There are many types of potential energy, each associated with a different type of force. This can be understood with the help of an example of the spring. When spring is displaced from its equilibrium position, it gains some amount of energy. It is observed that energy in the form of stress is felt on the hands upon stretching the spring. Thus, potential energy is the energy that can be associated with the arrangement of a system of objects that exert forces on one another.

elastic-energy-spring
Examples of potential energy
Batteries used enclose potential energy by separating charges that are excited towards each other. This potential energy can be converted into other forms of energy by completing the circuit.
The potential energy in food transforms into internal energy to maintain the body temperature of the body.
When the stretched bow is released, the arrow goes ahead with a large velocity on account of the potential energy of the stretched now.
![example-pt-energy]()
example-pt-energy
Potential energy can be stored in objects by compressing them, stretching them, twisting them, or bending them. But, potential energy can also be converted into other forms of energy by allowing the object to regain its original shape back.
The unit of potential energy (gravitational) is kg m²/s². It is same as the kinetic energy.
TYPES OF POTENTIAL ENERGY
Potential energy can be categorized into three types:-
GRAVITATIONAL POTENTIAL ENERGY
ELASTIC POTENTIAL ENERGY
ELECTRICAL POTENTIAL ENERGY
1. Gravitational potential energy:-
The gravitational potential energy of a body is defined as the energy possessed by the body to hold itself from the surface of the earth to a certain height against the gravitational force. When the body is allowed to fall from that heightened position, the amount of work done is equal to its gravitational potential energy.
Example of gravitational potential energy:- Water stored to great heights in dams is used to run turbines and generate electric energy at hydroelectric power storehouses.
To calculate the gravitational potential energy,
Let m = mass of the body, g = acceleration due to gravity on the surface of the earth, h= height at which body is raised.
Force applied to overcome gravitational attraction is
F= mg -(1)
Work done = force × distance -(2)
Putting the value of equation (1) into (2)
Distance moved is in the direction of force applied
So, W= F × h = mgh
W= mgh
This work gets stored as potential energy.
The Formula of Gravitational Potential Energy:- m × g × h
Points to remember about potential energy:-
Potential energy is for conservative forces. It does not occur for non-conservative forces.
Potential energy may be positive or negative.
A moving body may or may not have potential energy.
The formula of potential energy – PE= mgh
2. Elastic potential energy Elastic potential energy is the energy associated with the state of compression or expansion of adaptable objects such as spring, band, or rubber. An object that can stretch more can have more elastic potential energy.
Elastic potential energy can be evaluated using the formula:
U = ½kx²
Where U is elastic potential energy, k is spring force constant and x is string stretch length.
3. Electric potential energy
The electric potential energy refers to the total work done by an external agent in bringing the charges from infinity to the present arrangement without experiencing any acceleration. Energy is associated with the state of separation of two charged particles that interact electrically. In simple words, it is defined as the total potential energy a unit charge will acquire if placed at any point in outer space. It is a scalar quantity that possesses only magnitude but not direction.
The electric potential energy is measured in terms of joules and denoted by V.The dimensional formula is M1 L2 T-3 A-1
Theformulafor electric potential energy is given by
U = [1/(4πεo)] × [q1q2/d]
1. MECHANICAL ENERGY
Mechanical energy is the sum of kinetic energy and potential energy of the system. Kinetic energy and potential energy may differ from point to point but their sum is constant throughout.
The formula of mechanical energy is:- E= K+V
Let’s discuss it in more detail by an example of an ideal pendulum. When a pendulum swings back and forth, an exchange occurs between the kinetic and potential energy. The potential energy of the system becomes the highest when the bob attains its maximum height, whereas the kinetic energy becomes zero. The kinetic energy of the system becomes the highest when the bob attains the mean position and then the potential energy becomes zero. At both these extreme points, we can observe that the system possesses both kinetic and potential energy and the whole of which is constant. This also tells us about the conservation of mechanical energy.
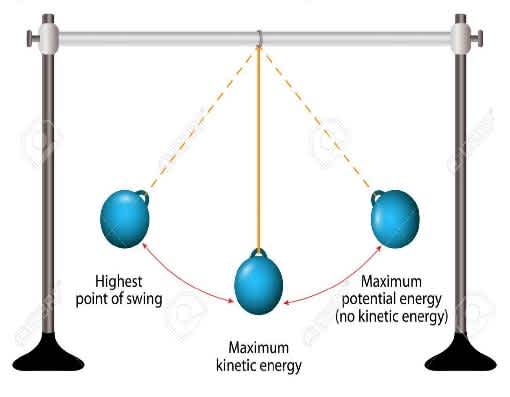
mechanical-energy
THE PRINCIPLE OF CONSERVATION OF MECHANICAL ENERGY
The principle of conservation of mechanical energy states that the total mechanical energy of a system is conserved if the forces doing work on the system are conservative or when the net work done by external non-conservative forces is zero.
To illustrate the law of conservation of mechanical energy, let’s calculate the kinetic energy K.E., potential energy P.E., and the total energy T.E. of a body falling freely under gravity.

principle-conservation-me
Let m = mass of the body at A, height = h.
The body is at rest at point A, therefore
At A, K.E of the body = 0
P.E. of the body = mgh
T.E. of the body = K.E. + P.E. = 0 + mgh
T.E. E1= mgh,
So E = mgh
The body is falling freely under gravity, let the velocity v when it strikes the ground at point C.
As we know, v² - u² = 2as we have, u = 0, a= g
v² - 0 = 2gh
v²= 2gh
At C, K.E. of the body = ½ mv² where v² = 2gh.
K.E. = ½ m (2gh)
K.E. = mgh
P.E. of the body= mgh where h = 0
So, P.E. = 0
T.E. = K.E.+ P.E.
T.E. = mgh + 0
So at C, E2 = mgh
In free fall, let the body cross any point B with a velocity v1, where AB =x
v² - u² = 2as
v1² - 0 = 2(g) x
v1² = 2gx
At B, K.E. of the body = ½ mv1² = ½m (2gx)
K.E. = mgx
Height if the body at B above the ground= CB = (h-x)
P.E. at B = mg(h-x)
Total energy at B = K.E.+ P.E.
E3 = mgx +mg(h-x)
E3 = mgx + mgh – mgx
E3= mgh
E1 = E2 = E3 = mgh
2. HEAT ENERGY
Heat energy is the energy possessed by a body by virtue of its random motion of the body. It is also associated with the force of friction. It is also known as thermal energy.
Examples of heat energy is
Heated swimming pools
Hot springs
3. INTERNAL ENERGY
Internal energy is the energy possessed by the body by virtue of the particular configuration of its molecules and also their motion. Internal energy is the sum of the kinetic energy and potential energy of the molecules of the system. In simple words, the total amount of energy possessed by a substance is internal energy.
Examples of internal energy are
Batteries
Compressed Gases
4. SOUND ENERGY
The vibrating particles of an object generate sound energy. Sound energy needs a medium to travel through. It does not travel in space or vacuum as there is no air as a medium.
Examples of sound energy are
Guitar strings
Beating drums
Tuning fork
5. ELECTRICAL ENERGY
Electrical energy is attained from the free moving electrons which are positively or negatively charged.
Examples of electrical energy are
Glowing bulb
Rotating fan
Ringing bell
MASS ENERGY EQUIVALENCE
The old saying was that mass and energy are two separate physical quantities, but as time passed Einstein made an incredible discovery that energy can be transformed into mass and mass can be transformed into energy.
The mass-energy equivalence relation given by Einstein is
E = mc²
Where m = mass that disappears, E = energy that appears, c = velocity of light in a vacuum
Mass and energy are not conserved individually but are conserved as a single entity called mass-energy.