Introduction
Let’s start this by asking a group of people that have anyone ever imagined how the reactions will take place? How will the atmosphere heats up in summer and cools down in winter? How do we have refrigerators that do the cooling part so well?
The answer all lies in thermodynamics. If we apply the thermodynamics applications in our routine life, then we can get the answers to every problem. People have heard this term quite a lot in newspapers and heard it on the television but they don't know anything about this. So, now we will start the thermodynamics with the basics of that to get a better understanding of what is and how the application of thermodynamics will help us in our day-to-day life.
Basic Concepts of Thermodynamics
basic-concept
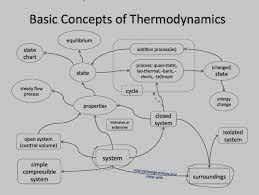
Thermodynamics has its vocabulary to deal with. A good understanding of this concept can lead to a sound understanding of concepts and can prevent the chances of having any misunderstandings.
1. System
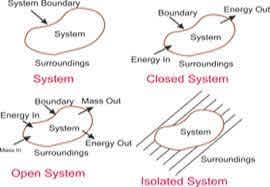
Thermodynamics is a system that is made up of a definite boundary on which the whole system will work. The system boundary can be either real or imaginary based on certain things. But the system boundary is very important to make the system more reliable and do the work. Some of the types which are there in this system are, Isolated Systems, Closed Systems, and Open Systems. All three types focus on transferring the energy between the systems.
2. Surrounding
Everything that is outside the system has a direct influence on the behavior of the systems because they hold the relationship between them. And that's why they are called surroundings.
Different Branches of Thermodynamics

branches-td
1. Classical Thermodynamics
In this branch of thermodynamics, the behavior of matter is an important element and also this will help to analyze the system in detail using the macroscopic technique. In this, units and temperatures are taken into consideration to know more about the process.
2. Statistical Thermodynamics
In this branch, molecules are taken very seriously and they have been put under the spotlight. In this, the main criteria to think about is knowing every property of molecules and also how they interact with each other can lead to understanding the behavior of these molecules.
3. Chemical Thermodynamics
This branch is different from the other two which are presented above because it talks about how work and heat are related to each other and how this will change into some reaction.
4. Equilibrium Thermodynamics
This branch talks about the study which takes into consideration the transformation of energy. The process focuses on getting the state of equilibrium to be stable.
Thermodynamics Equilibrium
equilibrium
All the properties in thermodynamics have a fixed value. And when one thing will change, the whole property will be changed too and so does the system’s value. In the system of equilibrium, no such changes will occur, even when the surroundings will get isolated.
The first thing to ensure here is that when the temperatures are the same throughout the entire process, the system is said to be in a thermal equilibrium state.
When there is no change in the pressure at any point, then the system will be considered to be in a mechanical equilibrium state.
When the chemical composition of a system does not change at any time due to anything, then the system will be called chemical equilibrium.
A last two-phase system focuses on the mass of each phase and when it reaches an equilibrium state it is called phase equilibrium.
Different Laws of Thermodynamics

laws-td
1. Zeroth law of thermodynamics
The Zeroth law of thermodynamics states that if two bodies are there in equilibrium with the third body in that, then they need to have a thermal equilibrium with each other. For example, let’s take two cups, cup A and cup B with the boiling water. There is a condition that when a thermometer is placed in cup A, after that it needs to get warmed up until it reaches 100 degrees celsius. And when the temperature reaches 100 degrees then we can say that the thermometer is in equilibrium to cup A.
2. The first law of thermodynamics
The first law of thermodynamics is also known as the law of Conservation of energy because by this law energy cannot be created nor destroyed. For example, plants convert the radiant energy to chemical energy through the process known as photosynthesis just like we use the energy to walk, run, swim, breathe, etc.
3. The second law of thermodynamics
The second law talks about the entropy in an isolated system. The point is to get the system to work so that it will increase the entropy of an isolated system. This system involves thermal equilibrium which is earned only when the state of maximum entropy of a system will increase. The reason behind this is that the entropy can increase or decrease at any point.
4. Third law of thermodynamics
The third law demands that the entropy system needs to approach a constant value. The entropy of a crystalline substance is at zero temperature at the zeroth point. This statement holds a lot of power with the minimum energy. For example, the molecules move freely without any stress here and there and also they have high entropy.
Applications of the second law of thermodynamics
By this law, the heat always flows from the higher temperature to the lower temperature. This applies to all the types of heat engines whether it is diesel, petrol, fluids, etc. The law is very important in real life and can lead to progress with the days.
The next application of thermodynamics talks about heat pumps which can be reversed in the Carnot cycles specifically.

entropy
In an original Carnot cycle, heat produced can reverse the working of the Carnot cycle and provide the best way to transfer the heat from lower to higher temperatures.
To remove the heat from the food items, suppose they are kept in the refrigerator, and throwing them away will not change the temperature automatically. In this, we need to acquire the external work which we can get from the compressor to make this happen in a refrigerator.
Other applications of Thermodynamics
In a room where there are so many people and they are sweating, it happens because the body will start cooling down by transferring the heat to the sweat. And after that, the sweat evaporates by adding the heat to the room.
When we put some ice- cubes in a liquid, the ice- cubes will start melting because the liquid will start absorbing the heat of ice- cubes from the liquid and making the drink cooler.
A person will require the mental and the emotional stimulation from the environment, here the entropy can work the best.
Entropy is used to measure the things which are falling apart in a real-life. When the entropy is greater, then it will be fast for the deterioration of life.
Thermodynamics Equations
The equation of a state of the substance provides the additional information which was needed to calculate the amount of work that has been used in making the transition from one equilibrium state to another depending upon the specified path. The equation of a state is highly expressed as a functional relationship that has been used to connect the various parameters which are a very important element in making the transition take place.
The basic concepts of the thermodynamic system are here to discuss the simple gas which is necessary inside of a cylinder to move as per the piston which moves around.
The ideal gas low is a key to finding the independence of one equation over the other so the formulae are PV = nRT, here ‘n’ is the number of moles which is present inside the gas, ‘R’ means the universal gas constant and ‘K’ is 8.3145 joules.
Conclusion
In the end, we can only say that according to the first law of thermodynamics, the internal energy is directly proportional to its temperature. And when the work is done in the system, the heat will start flowing to the outside, and when this process takes place then the heat will start flowing to the inside of the system. The energy that can be created and destroyed depends upon the temperature to be used and the process on which all the systems will de[pens. One thing that needs to be ensured is that the system has to be equal to the energy when the process leaves the system.