Introduction
Why does solid wood burn and becomes ash smoke and gases? all of which spread energy outwards more easily than the solid fuel? Ice melting, salt, sugar or ink dissolving, making popcorn and boiling water for tea are processes we see in daily life that occur with a different kind of a phenomenon but why? To answer this question German physicist Rudolf Clausius 1850, highlighted the concept of Entropy.But it was a young French engineer, Sadi Carnot (1796–1832), who first went for the idea of thermodynamic efficiency; however, the idea was so fresh and new to people at that time that it had little impact.
Introduction
Terms you need to know before understanding entropy.
Spontaneous Process – This term generally refers to the process which happens by itself or due to very little external force.In nature, Spontaneous Processes occur due to increased disorderliness or randomness
Non- Spontaneous Process– Process which does not occur by itself which means Randomness is negligible.
Isolated and Non- Isolated system.
Reversible and irreversible Process.
Adiabatic process - when a physical proces
s in a system takes place in such a way that there is no exchange of heat between the system and the atmosphere, the process is called the Adiabatic process.
Why is there a need for Entropy?
We need to study Entropy to have a better understanding of the Spontaneous behavior of a process and the direction of Spontaneous change. We will have an insight into why things naturally happen in a random direction or a disordered manner.
To procure a quantitative estimate for the direction of random change, Clausius brought up the concept of entropy as a mathematical way of expressing the second law of thermodynamics. The Clausius form of the second law states that spontaneous change for an irreversible process in an isolated system (that is, one that does not exchange the heat or work with its surroundings) always proceeds in the principle of increasing entropy. We need to study Entropy to have a better knowledge of the Spontaneous behavior of a process and the direction of Spontaneous change. We will have an insight into why things naturally happen in a random direction or a disordered manner on both a macro and a microscopic level.
What exactly does the term Entropy mean? And how did it originate?
Entropy is a concept that is based on going through the spontaneity or randomness of a process or a reaction. In simple words, To know whether a process is spontaneous or not we use entropy, but "you can't measure Randomness" that how random or disordered a thing is? But we can measure the Change in Entropy, denoted by, "∆S".

Entropy-mean
More disordered, more ENTROPY, less disordered, less entropy.
How to measure Entropy?
If a process is Spontaneous then the Entropy will increase, that is ∆S = +ve.
S final–S initial = ∆S
Here when final entropy is greater than initial entropy, after subtraction we get positive ∆S and "∆S > 0 ".
2. When the process is nonspontaneous entropy becomes negative as randomness is decreased or becomes negligible.
"∆S < 0"
3. When a process works from A→B as well as from A←B spontaneously the entropy becomes zero.
"∆S = 0"
4. Entropy is a state function
5. Change in entropy does not depend upon the path through which the change has occurred.
6. ∆S = S Product – S Resultant in case of Chemical reaction. 7. Entropy shows an extensive property that it is mass, amount of matter, and mole dependent.
8. A accurate formula for entropy is –: ∆S = ∆Q
[: T For constant temperature.
On gaining heat, ∆S = +ve
On losing heat, ∆S = – ve] 9. When the temperature is not constant –
ds = dq
T: On integrating it -∆S = ∫dq
10. Unit of Entropy -
J/molK.
11. For an Isolated system, due to the non-transferrable nature of the system for heat and mass
∆Q = 0
But if it is showing spontaneous behavior and has increased randomness then -
∆S = ∆Q {Here, ∆Q= 0}
T
∆S ≠ 0
As entropy is increased in the system.
For example - There are two containers separated by a valve. The containers are made up of an insulating layer.
If we open the valve the gases will mix spontaneously and can't be separated spontaneously.
Due to the insulation of the container, there is no transfer of heat or mass, so ∆Q will become zero but due to the Spontaneous process Entropy will increase and " ∆S > 0".
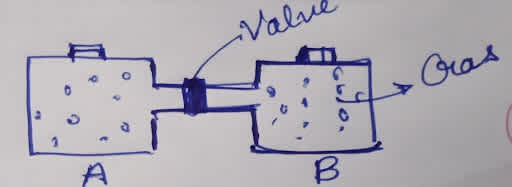
Isolated system Entropy
12. For a Non-isolated system
∆SSystem + ∆SSurrounding = ∆Suniverse
Here change in Entropy of surrounding matter the most as it increases by a huge amount. Sometimes ∆S of the system could be negative but due to ∆S of the surrounding >> 0 it makes,
∆S of system + ∆S of surrounding =∆S of universe > 0
13. Entropy is an extensive property which means that it measures the size or extent of a system. It can also be considered a comprehensive property
What are the real-life applications and examples of Entropy?
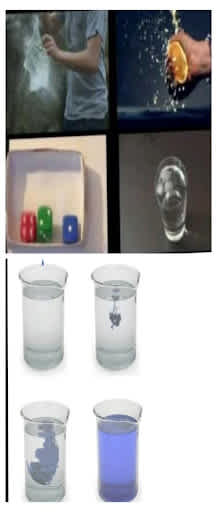
Some of the examples of entropy from everyday life are campfires, Ice melting, salt or sugar dissolving, popcorn making, and boiling water. Entropy also measures the heat per temperature and thermal energy.
Water has to release heat to become Ice which means ∆S system = –ve ∆S system < 0
So will this process take place? Yes, as ∆S of surrounding >> 0, it will make ∆S of universe > 0.
This means that the conversion of water into ice is not about ∆S of system > 0, it's about ∆S ofuniverse > 0.
Entropy of Fusion
It is the gain in entropy when a solid melts into a liquid. The entropy rises as the freedom of movement of molecules increases with phase change. The entropy of fusion is equal to the enthalpy of fusion divided by melting point (fusion temperature) ∆fusS=∆fuse / Tf A natural process such as a phase change (eg. fusion) will occur when the correlated change in the Gibbs free energy is negative. Most of the time ∆fusS is positive Exception Helium-3 has a negative entropy of fusion at temperatures below 0.3 K. Helium-4 also has a very little negative entropy of fusion below 0.8 K.
Entropy of Vaporization
When there is an increase in entropy as liquid changes into vapour then it is called the entropy of vaporization is a state. This is due to a rise in molecular movement which builds randomness of motion. The entropy of vaporization is equal to the enthalpy of vaporization divided by the boiling degree. It can be represented as; ∆vapS = ∆vape / Tb
Conclusion
In one statistically significant entropy, it is found that for a very large system in thermodynamic equilibrium, entropy S is proportional to the natural logarithmic quantity Ω representing the maximum number of microscopic ways in which the macroscopic state conforming to S can be known; that is, S = k ln Ω, in which k is the Boltzmann constant that is related to molecular energy. All spontaneous processes are irreversible; hence, it has been explained that the entropy of the universe is increasing: that is, more and more energy becomes inaccessible for conversion into work. Because of this, the universe is confided to be “running down.” Some things are contrary to expectations about entropy. An unboiled egg has lesser entropy than a hard-boiled egg. It is because of the denaturation of the secondary structure of the protein. The helical structure into a random coiled form is Protein changes. If we stretch a rubber band, entropy drops because macromolecules get uncoiled and arranged in a more ordered manner. Therefore randomness will decrease.
