Milk appears to be a homogeneous mixture because it seems to have a uniform distribution of particles throughout. But when examined under a microscope, milk consists of tiny substances. Milk is composed of globules of fats and proteins dispersed in water. That is why milk is a heterogeneous mixture. The components of such a mixture can be separated by simple means. Mixtures that sometimes seem to exist as homogeneous are often found to be heterogeneous after microscopic examination.
This blog deals with components of milk responsible for its homogeneous or heterogeneous behavior. Here, you can know What homogeneous mixtures and Heterogeneous mixtures are? And their properties. Let’s study it in more detail.
What are mixtures?
Mixtures are prepared by mixing two or more substances that do not combine chemically with each other. This simply means that the substances do not lose their individuality. Each component mixed keeps its original properties. The separation of the components can be done easily by different physical methods. Almost everything we see around is made up of a mixture of two or more substances. An example of a mixture is air. Air is a mixture of gases like oxygen, carbon dioxide, nitrogen, and many more.
Types of Mixtures:
There are two essential types of mixtures-
Homogeneous mixture
Heterogeneous mixture
Homogeneous Mixture:
Homogeneous mixtures are those mixtures in which substances are entirely mixed and are indistinguishable from one another. All homogeneous mixtures are called solutions because the components are uniformly mixed throughout the mixture. Only one phase of material can be observed. This mixture has a uniform composition and has no visible boundaries of separation.
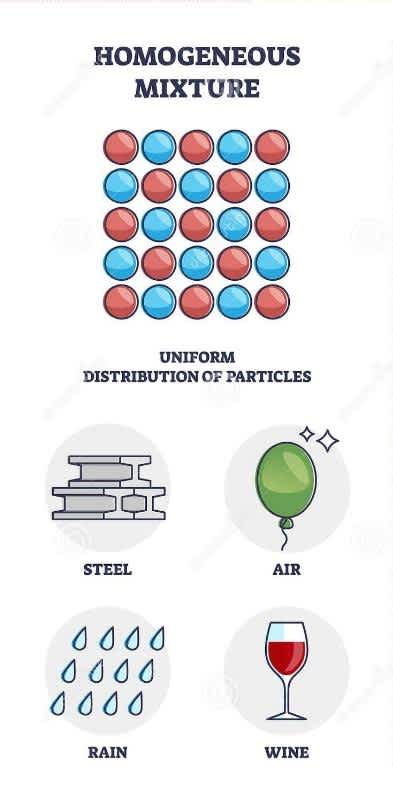
Homogeneous mixtures
Properties of a Homogeneous Mixture
All solutions are an illustration of homogeneous mixtures.
The size of the particles in a homogeneous mixture is smaller than one nanometer.
The boundaries of separation of the two components are not visible.
The method to separate the components of a homogeneous mixture can be difficult.
The components of the mixture are may be present in unequal proportions.
Components are not apparent to the naked eye.
Heterogeneous Mixture:
Heterogeneous mixtures are those mixtures in which substances do not combine properly and are distinguishable from one another. The composition of components occurs non-uniformly throughout the mixture. The components of the mixture are present in different phases or more than one phase. That is why the boundary of separation is easily visible.
Simply, we can say that the components present in a heterogeneous mixture don’t dissolve completely into each other and don’t lose their identity either. We can easily recognize a heterogeneous mixture through our naked eyes.
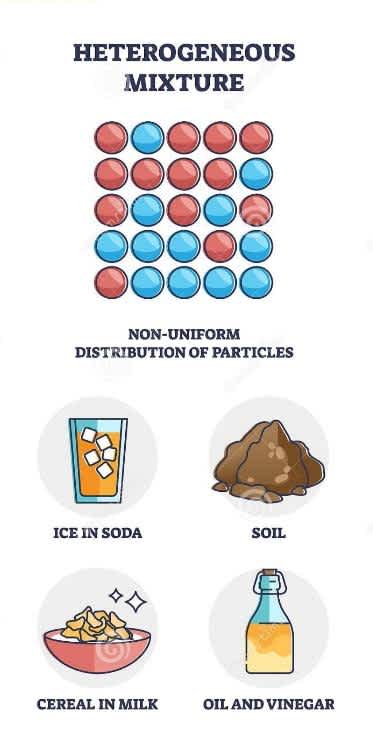
Heterogeneous mixtures
Properties of a Heterogeneous Mixture:
A heterogeneous mixture contains more than one phase or ingredient.
The components remain physically separate.
The separation is easily visible in such mixtures.
The samples taken from the different places of the mixture may have a different composition.
There are many easy methods to separate the components of the mixture.
The components are apparent to the naked eye.
Activity & Examples
You may try this activity at home to understand the difference between homogenous and heterogeneous mixtures:-
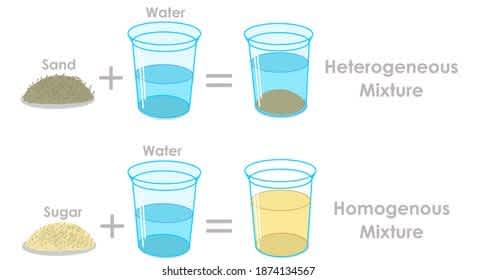
ACTIVITY TO IDENTIFY MIXTURES
The mixture of salt in water is an example of a homogeneous mixture. Let’s see how? Take a glass full of water and add one teaspoon of salt to it. You will observe that the two things mix so well into each other that we cannot even separate them by any physical method. The boundary of salt and water cannot be differentiated in this mixture. This shows that the mixture is homogeneous.
The mixture of sand in water is an example of a heterogeneous mixture. Take another glass of water and now add sand to it. You will observe that the two components do not mix that well. Wait for 2-3 minutes and observe that the sand particles will start settling down. The boundary of separation of the two components is easily visible by naked eyes. This shows that the mixture is heterogeneous.
You will learn the difference between homogeneous and heterogeneous mixtures after this activity.
Difference between homogeneous and heterogeneous mixture:-
Homogeneous Mixture
It has a uniform composition throughout the mixture.
The components of the mixture are not apparent to the naked eyes.
The whole mixture is in the same phase.
The separation of components is difficult.
Examples:- Soft drinks, soda water, saltwater, or air.
Heterogeneous Mixture
It has a non-uniform composition throughout the mixture.
The components of the mixture are apparent to the naked eyes.
The mixture may have two or more phases.
The separation of components is easy and can be done by simple methods.
Examples:- Sand water, beach sand, petrol in water, milk, many more.
Do You Know?
Milk is an example of a heterogeneous mixture. How is this possible? Milk that we buy has a uniform composition throughout and it does not even separate upon standing. So, it should be a homogenous mixture? Then how is it a heterogeneous mixture?
Why Milk is a homogeneous mixture?
Milk appears to be a homogeneous mixture. The components of pure milk which are nutrients (fat and protein) present in it, don’t form separate layers. And hence they can't be separated by physical methods.
After examination under a microscope, whole milk is found to have globules of fats and proteins dispersed in water. Milk contains fat and water together which are two immiscible liquid phases. So, another reason could be the fact that the fat and the water components are immiscible. They can not form a solution together. That is why milk is a heterogeneous mixture. The components of a heterogeneous mixture can be separated by simple means.
Various physical methods can be used to separate cream from milk (centrifugation). When we even heat milk at a high temperature, a fine layer of fat and coagulated proteins form on the surface of milk which can then be removed. There are many types of physical methods to separate milk from its components which can prove that milk is heterogeneous.
All these examinations show that milk is a heterogeneous mixture.